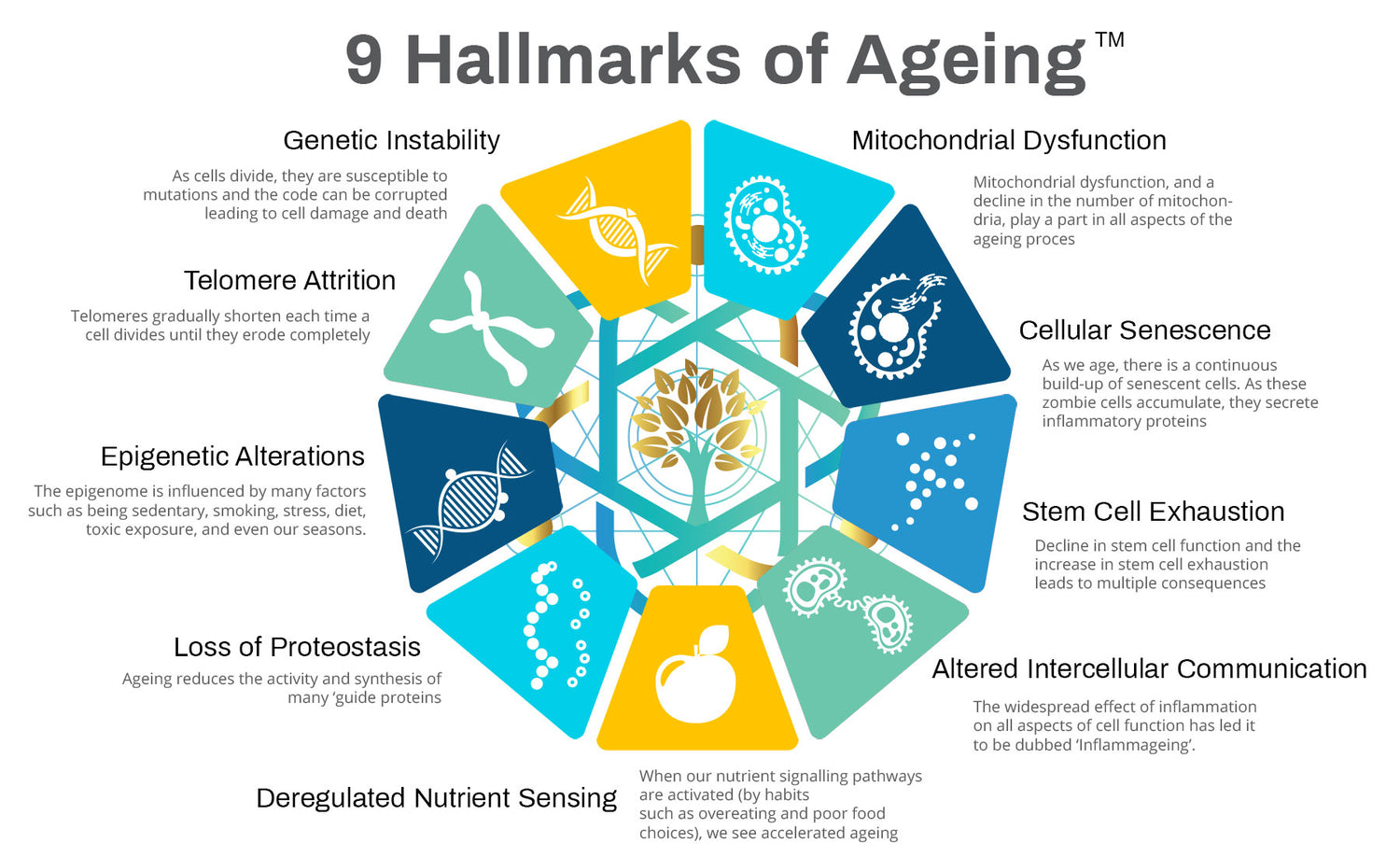
Genetic Instability
The genome is the collection of all the genetic information of a person or species - it is the map on how to build the whole organism. This genetic material is called the CODE and it is vital to protect the integrity of this information at all costs.
As cells divide, they are susceptible to mutations and the code can be corrupted leading to cell damage and death. They also get damaged by free radicals and environmental toxins.
To prevent gene instability, our DNA have repair mechanisms
to protect the code and to correct corrupted DNA. This is achieved by reprogramming abnormal cells and boosting mitochondrial function and numbers via enhancing stem cell activity, boosting NAD production, and activating Nrf2 gene defences.
“We must defend our genes at all costs to preserve the ‘life code’ they contain.”
Telomere Attrition
Our chromosomes, located in the cell nucleus, carry much of our genetic material. The area on the ends of our chromosomes are called telomeres and are often described as being similar in function to plastic tips on the end of your shoelaces.
These telomeres gradually shorten each time a cell divides
until they erode completely and leave the DNA in your chromosome exposed and open to damage which can lead to cell death or mutations and cancerous changes.
Scientists have proven that telomeres are critically important in the life expectancy of a cell. Vital enzymic mechanisms such as ‘telomerase’
have a large part to play in rejuvenating and regenerating damaged chromosomes and extending life.
“Protecting the end caps of our chromosomes and supporting their repair is crucial to longevity.”
Epigenetic Alterations
The study of epigenetics is foundational to our understanding, and influence, on the ageing process. Epigenetics describes the mechanisms that control the expression of our genes. Genes contain the life CODE and epigenetics influence the use of our genes – when genes switch on or off, when they share their information, and how they signal surrounding cells.
Our epigenome is the environment that surrounds all our
chromosomes and can modify and trigger the expression of all our genes.
The epigenome is influenced by many factors such as being sedentary, smoking, stress, diet, toxic exposure, and even our seasons. However, scientists have found that our epigenetics can be improved and reversed, which leads to correct expression of our genes.
“We can switch genes on, and we can switch genes off – our epigenetics control our gene expression.”
Loss of Proteostasis
Proteins, and the amino acids they are made of, are the building blocks of life. They give structure to all our cells and act as hormones, as antibodies, as well as carrying messages between all our cells.
Ageing reduces the activity and synthesis of many ‘guide’
proteins leading to a mismanagement of protein roles, assembly problems, transport issues, and even the death of master proteins.
Researchers have discovered that our mitochondria play a
vital role in preventing the mismanagement of our cellular proteins and that ATP, an energy source for the human body, can improve the behaviour of, and communication amongst, all our proteins.
“Keeping our mitochondria healthy leads to correct protein management within our cells.”

Deregulated Nutrient Sensing
Our bodies have a vast array of receptors that receive and transmit signals to communicate with and activate genes and pathways. These are known as
our intracellular and extra cellular signalling pathways. One very well known intracellular pathway, or nutrient sensing receptor, is IGF-1, which is activated by insulin and informs the cell of the presence of glucose.
Researchers have discovered we have many age-controlling
signalling pathways with some of the most well known being the FOXO family, mTOR complexes, Sirtuins, and Akt pathways – all of which play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.
When our nutrient signalling pathways are activated (by habits
such as overeating and poor food choices), we see accelerated ageing occur but by inhibiting these nutrient signalling pathways (signalling nutrient scarcity and calorie restriction), we can extend our longevity.
“Longevity genes have pathways that need to be regulated by our nutrition and other chemical signals.”
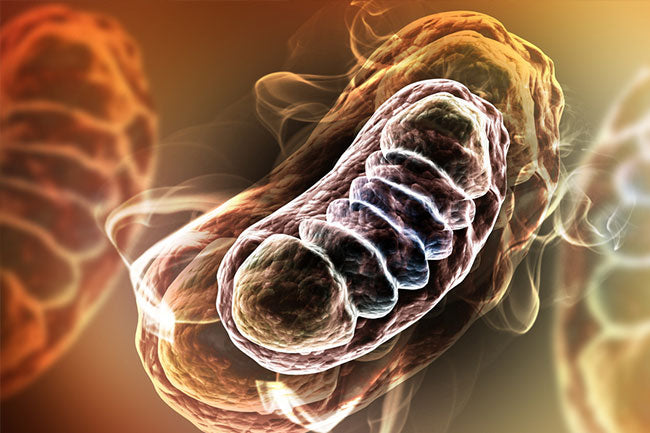
Mitochondrial Dysfunction
Mitochondria are known as the powerhouse of the cell; these
organelles play a vital role in the function of every cell and organ in the human body. The role of these powerhouses is to take the food that we eat and the air we breathe, and chemically convert them into energy rich molecules called ATP (adenosine triphosphate).
Their function is vital, and mitochondria have a profound
impact on ageing. Mitochondrial dysfunction, and a decline in the
number of mitochondria, play a part in all aspects of the ageing process, in age-related disease development, and can also lead to the premature death of the cell.
Mitochondrial alterations are a complex phenomenon – with deterioration, dysfunctional cell-mitochondrial communication, mtDNA mutations, and oxidative stress, all being mechanisms that can negatively affect the activity and number of our cellular powerhouses.
As our mitochondria decline in function, we are left tired, debilitated, and experiencing accelerated ageing. Upregulating the mitochondrial response and regrowing new mitochondria can therefore regulate our longevity and improve our healthy years.
Mitochondrial function is key to longevity – protect them, repair them, regenerate them.
Cellular Senescence
Senescence is a process by which a cell ages and permanently stops dividing but does not die. These cells are often referred to
as ‘zombie cells’ as they don’t die and continue to cause damaging effects to the healthy cells around them.
While cellular senescence is a beneficial response to damaged
cells, cancer, and wound healing, and is also a healthy part of early development, it becomes harmful as more and more cells lose their function and our tissue exhausts its regenerative capacity.
As we age, there is a continuous build-up of senescent cells. As these zombie cells accumulate, they secrete inflammatory proteins
that damage the surrounding cells and become a burden on our autophagy mechanisms to mop up and dispose of those useless cells. We rely on an efficient immune system and robust stem cell activity to regulate and reduce this accumulation.
This is called Senolytic activity - clearing away the debris that is clogging up every part of our body.
Clear out the debris that clogs up our tissue – ‘zombie cells’ cause chronic disease and rapid ageing.
Stem Cell Exhaustion
One of the most obvious characteristics of ageing is the
decline in the ability of our tissue and organs to regenerate. We see increasing and rapid changes in the health of our face and hair, we lose muscle tone and become frail, and we experience more chronic disease.
Stem cells are responsible for the renewal of cells in an organ and all body tissue, they are stored in the body and used when needed. With ageing, our tissue and cells do not get replaced or repaired very well due to the slowdown of cell division and the lack of stem cell replacements.
This decline in stem cell function and the increase in stem cell exhaustion leads to multiple consequences such as an accumulation of defective, pre-cancerous cells, muscle loss and organ degeneration. The lack of stem cells inhibit the cells renewal process and the body’s ability to repair itself and function efficiently.
Build our stem cell pool and boost their circulation to damaged tissues requiring repair.
Altered Intercellular Communication
Our cells have multiple signalling pathways they use to communicate and interact with one another. The most prominent ones being the
endocrine, neuroendocrine, and neuronal pathways. Any change or alteration of these signals/messages leads to a breakdown in the cell and a decline in tissue health.
There are many factors that can cause this decline and interfere
with cell signalling. Scientists have discovered that our cellular
communication is disrupted and negatively impacted by the age-related dysfunctions occurring in all the other Hallmarks of Ageing - like ripples in a pond.
Inflammation is one of the single major factors influencing
all the Hallmarks and has also been a major focus of study on how it causes alterations in cell-biology and disrupts our cellular communication. The widespread effect of inflammation on all aspects of cell function has led it to be dubbed ‘Inflammageing’. Unopposed inflammation impacts cells via damage to receptor sites and glandular tissue, inhibiting hormone secretions, driving defects in our DNA, down-regulating gene expression, and reducing the diversity of our microbiome.
Inflammation is the silent killer, and its reduction is at the core of all anti-ageing therapies.